Definition Of Buffer In Chemistry
In chemistry buffer solution and examplesIt is a solution containing either a weak acid and its salt or a weak base and its salt which resists changes in pH. A buffer is an aqueous solution used to keep the pH of a solution nearly constant.
 Acid Base And Buffer Chemistry Youtube
Acid Base And Buffer Chemistry Youtube
A buffer is a solution that can resist pH change upon the addition of an acidic or basic components.
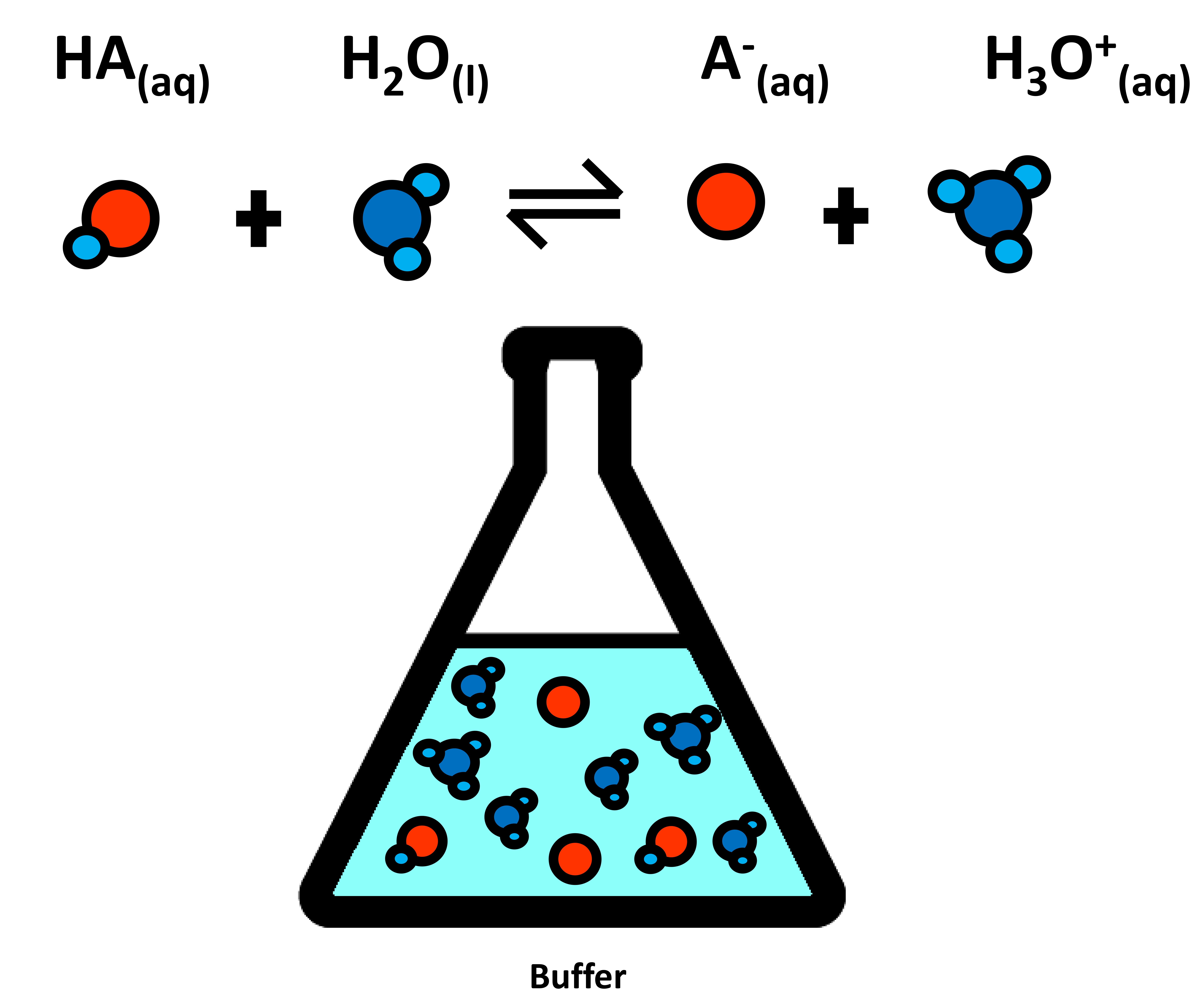
Definition of buffer in chemistry. In water solution sodium acetate is completely dissociated into sodium Na and acetate CH 3 COO - ions. In nature there are many systems that use buffering for pH regulation. Buffers are used to make solutions of known pH especially for instrument calibration purposes.
To resist a change in pH if H increases in a solution the buffer will _____ H. The higher the acid concentration of the buffer then the buffer capacity will be higher as well. A buffer solution can be made by mixing a weak acid with one of its salts OR mixing a weak base with one of its salts.
A buffering agent is a weak acid or weak base that helps maintain the pH of an aqueous solution after adding another acid or base. Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes. You can explore more about buffer solutions here.
Buffers in a solution typically consist of an acidbase pair. Buffers are extremely useful in these systems to maintain the pH at a constant value. In other words a buffer is an aqueous solution of a weak acid and its conjugate base or a weak base and its conjugate acid.
Here we are going to learn about buffer capacity chemistry definition and formula. A buffer is an aqueous solution that consists of a mixture of a weak acid and its salt acid buffer or a weak base with its salt basic buffer. Buffer in chemistry solution usually containing an acid and a base or a salt that tends to maintain a constant hydrogen ion concentration.
Now Buffer Capacity can be defined as the measure of the efficiency of a buffer in resisting its change in pH. An example of a common buffer is a solution of acetic acid CH 3 COOH and sodium acetate. A buffer is a solution that can resist pH change upon the addition of an acidic or basic components.
A buffer solution refers to an aqueous solution. How are Acid-Base Buffers Made. The buffer capacity can also be defined as the amount of mole of strong base needed to change the pH of 1 L of solution by 1 pH of unit.
By definition a buffer system is a solution that resists a change in pH when acids or bases are added. This does not mean that the pH of buffers does not change. This solution is quite important in the field of chemistry.
It is able to neutralize small amounts of added acid or base thus maintaining the pH of the solution relatively stable. Buffer bŭf ər Chemistry A substance that prevents change in the acidity of a solution when an acid or base is added to the solution or when the solution is diluted. It is a solution in water of a mixture of a weak acid or base and its salt.
A lot of biological and chemical reactions need a constant pH for the reaction to proceed. Buffers are solutions that resist a change in pH on dilution or on addition of small amounts of acids or alkali. A buffer is a compound that resists changes in pH when a limited amount of acid or base is added to it.
Its pH changes very little when a small amount of strong acid or base is added to it and is thus used to prevent a solution s pH change. It is able to neutralize small amounts of added acid or base thus maintaining the pH of the solution relatively stable. Acidic solutions contain high concentrations of hydrogen ions H and have pH values less.
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa. The buffer capacity is a quantity in resisting the pH change at the time of addition of an acid or base. Ions are atoms or molecules that have lost or gained one or more electrons.
To resist a change in pH if H drops in a solution the buffer will _____ H. If you add an acid or a base to a buffered solution its pH will not change significantly. Buffer definition is - fellow man.
This is important for processes andor reactions which require specific and stable pH ranges. Furthermore it consists of a mixture of a weak acid and its conjugate base or vice-versa. For example the bicarbonate buffering system is used to regulate the pH of blood.
A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. A buffer is an aqueous solution that has a highly stable pH.
Its pH changes very little when a small amount of strong acid or base is added to it. The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. A buffer is a solution that can maintain a nearly constant pH if it is diluted or if relatively small amounts of strong acids or bases are added.
Definition of a buffer. How to use buffer in a sentence. This is important for processes andor reactions which require specific and stable pH ranges.
A buffer solution is chemical solution which resists change to its pH or acidity. The pH of the solution changes very little when a small amount of strong acid or base is added to it.
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Solutions Electron Configuration
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Solutions Electron Configuration
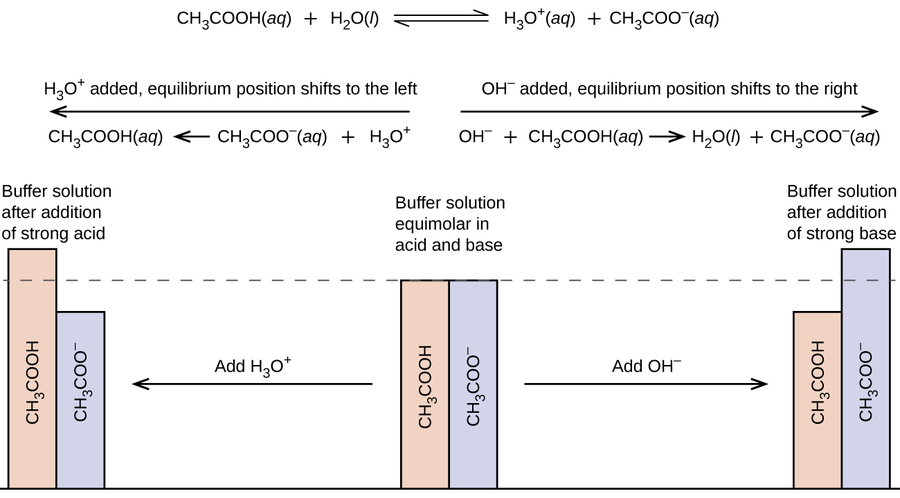 7 1 Acid Base Buffers Chemistry Libretexts
7 1 Acid Base Buffers Chemistry Libretexts
 What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
 Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
 Buffer Solution Its Characteristics Types And Preparations
Buffer Solution Its Characteristics Types And Preparations
 Henderson Hasselbalch Equation Microbe Notes
Henderson Hasselbalch Equation Microbe Notes
 Common Ion Effect And Buffers Video Khan Academy
Common Ion Effect And Buffers Video Khan Academy
Buffer Solution Solution Of Reserve Acidity Alkalinity
 Buffers Introductory Chemistry
Buffers Introductory Chemistry
 Introduction To Buffers Chemistry Libretexts
Introduction To Buffers Chemistry Libretexts
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
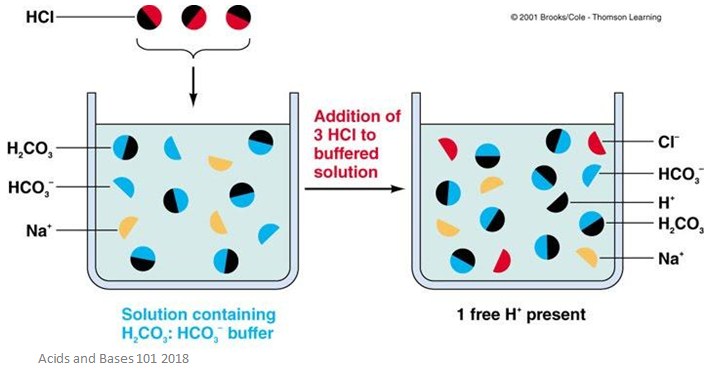 Acid Base Buffers Facts Summary Definition Chemistry Revision
Acid Base Buffers Facts Summary Definition Chemistry Revision
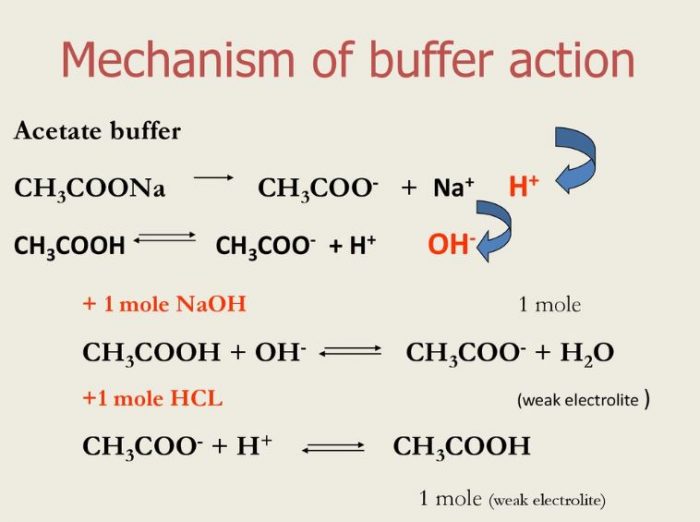 Buffer Solution And Buffer Action Chemistry Class 11 Ionic Equilibrium
Buffer Solution And Buffer Action Chemistry Class 11 Ionic Equilibrium
 Buffer Solution Ph Calculations Video Khan Academy
Buffer Solution Ph Calculations Video Khan Academy