Buffer Definition Biology Quizlet
Now Buffer Capacity can be defined as the measure of the efficiency of a buffer in resisting its change in pH. An area separating land used for different purposes a buffer zone between the development and the park.
 Balancing Redox Reaction By Oxidation Number Change Method Redox Reactions Oxidation Reactions
Balancing Redox Reaction By Oxidation Number Change Method Redox Reactions Oxidation Reactions
Its pH changes very little when a small amount of strong acid or base is added to it.

Buffer definition biology quizlet. If you add an acid or a base to a buffered solution its pH will not change significantly. Chemistry A substance that prevents change in the acidity of a solution when an acid or base is added to the solution or when the solution is diluted. What Is a Buffer.
Learn buffer biology with free interactive flashcards. An example of a common buffer is a solution of acetic acid CH 3 COOH and sodium acetate. What is Buffer in Biology.
Ions are atoms or molecules that have lost or gained one or more electrons. A buffer is an aqueous solution that has a highly stable pH. Aaron Rodgers and Shailene Woodley are engaged and we couldnt be happier.
Water is the most important biological solvent. Minimizes fluctuations in pH. Buffer solutions are used as a.
Legal Definition of buffer zone. Buffer in chemistry solution usually containing an acid and a base or a salt that tends to maintain a constant hydrogen ion concentration. 0-6 is acidic 7 neutral and 8-14 basic.
Biology I - Chapter 2-1. In this way a biological buffer helps maintain the body at the correct pH so that biochemical processes continue to run optimally. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt which is resistant to changes in pH.
Acids bases pH and buffers. Buffer - Definition and Examples - Biology Online Dictionary A buffer solution more precisely pH buffer or hydrogen ion buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa. In other words a buffer is an aqueous solution of either a weak acid and its conjugate base or a weak base and its conjugate acid.
Biology Dictionary tris EDTA TE solution and NaCl TNE buffer is a buffer solution used in molecular biology especially for DNA and RNA Solution Definition and Examples Biology Online Dictionary Examples of Solvent Water. An area designed to separate. An area with certain boundaries beyond which protestors may not pass.
Choose from 498 different sets of buffer biology flashcards on Quizlet. Buffers are used to make solutions of known pH especially for instrument calibration purposes. Its pH changes very little when a small amount of strong acid or base is added to it.
This is the currently selected item. Take A Sneak Peak At The Movies Coming Out This Week 812 Its official. Most buffers consist of a weak acid and a weak base.
Measurement scale for hydrogen ion concentration. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. An acid-base balancing or control reaction by which the pH of a solution is protected from major change when acid or base is added to it.
Acids bases and pH. Molecules tending to lower the hydrogen ion concentration in a solution and raise its pH numerically. Buffer a weak acid or base that can react with strong acids or bases to help prevent sharp sudden changes in pH THIS SET IS OFTEN IN FOLDERS WITH.
Biology I - Chapter 2-2 Flashcards Quizlet. Click to get the latest Buzzing content. Acids bases and pH.
In water solution sodium acetate is completely dissociated into sodium Na and acetate CH 3 COO - ions. The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. Comments on buffer zone.
Reactants are added to a solution which way is the equilibrium driven. Buffer solutions are used as a. Similarly adding water to a buffer or allowing water to evaporate will not change the pH of a buffer.
Biology is brought to you with support from the Amgen Foundation. A buffering agent is a weak acid or weak base that helps maintain the pH of an aqueous solution after adding another acid or base. Acids bases and pH.
The protection is afforded by the presence in the solution of a weak acid and related salt for example acetic acid and sodium acetate which maintains the equilibrium by means of ion transfer and neutralization. A buffer may also be called a pH buffer hydrogen ion buffer or buffer solution. Substance or group of substances that tend to resist pH changes of a solution thus stabilizing its relative acidity and basicity.
Start studying pH and buffers principles of biology. A buffer is a compound that resists changes in pH when a limited amount of acid or base is added to it. Learn vocabulary terms and more with flashcards games and other study tools.
Entropy always increases in an isolated system. Biology is brought to you with support. There are two key terms associated with buffers.
Learn vocabulary terms and more with flashcards games and other study tools. Start studying Biology Buffers. Online Library Buffer Solution Definition Biology Buffer Definition and Examples Biology Online Dictionary A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa.
 Biology Chapter 2 Flashcards Quizlet
Biology Chapter 2 Flashcards Quizlet
 Quizlet 12 Ways To Go Beyond The Basic Vocab List Vocab Learn Spanish Online How To Speak Spanish
Quizlet 12 Ways To Go Beyond The Basic Vocab List Vocab Learn Spanish Online How To Speak Spanish
 Biology Buffers Flashcards Quizlet
Biology Buffers Flashcards Quizlet
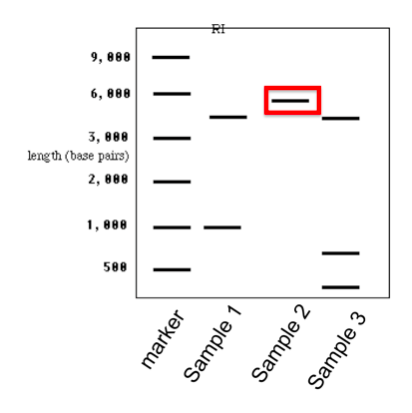 Bio 140 Lab Gel Electrophoresis Prelab Quiz Diagram Quizlet
Bio 140 Lab Gel Electrophoresis Prelab Quiz Diagram Quizlet
 Small Intestine Histology Labeled Google Search Human Anatomy And Physiology Medical Knowledge Biology Worksheet
Small Intestine Histology Labeled Google Search Human Anatomy And Physiology Medical Knowledge Biology Worksheet
 Ap Biology Semester 1 Final Review Flashcards Quizlet
Ap Biology Semester 1 Final Review Flashcards Quizlet
 Chapter 3 Acid Base Chemistry And The Limits Of Biological Life Flashcards Quizlet
Chapter 3 Acid Base Chemistry And The Limits Of Biological Life Flashcards Quizlet
 Cell Compounds And Biological Molecules Flashcards Quizlet
Cell Compounds And Biological Molecules Flashcards Quizlet
 Diagram Of Protein Electrophoresis Workflow Study Biology Clinical Chemistry Microbiology Study
Diagram Of Protein Electrophoresis Workflow Study Biology Clinical Chemistry Microbiology Study
 Balancing Redox Reaction By Oxidation Number Change Method Redox Reactions Oxidation Reactions
Balancing Redox Reaction By Oxidation Number Change Method Redox Reactions Oxidation Reactions
 What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
 Biology Next Step Flashcards Quizlet
Biology Next Step Flashcards Quizlet
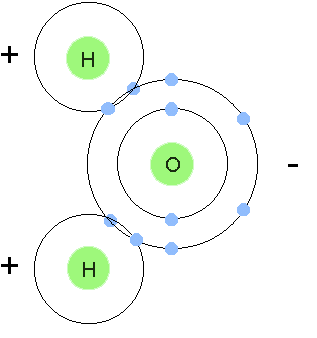 Biology Chapter 3 Flashcards Quizlet
Biology Chapter 3 Flashcards Quizlet
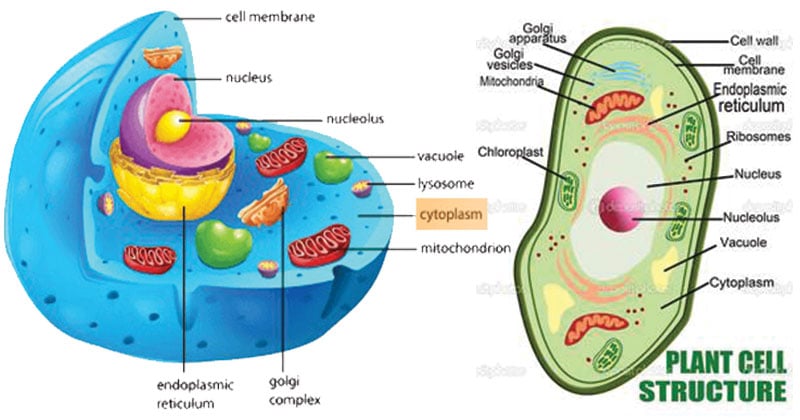 Cytoplasm Definition Structure Functions And Diagram
Cytoplasm Definition Structure Functions And Diagram
 Ap Bio Unit 1 Answers Flashcards Quizlet
Ap Bio Unit 1 Answers Flashcards Quizlet
 Biology Part 4 Chemistry Flashcards Quizlet
Biology Part 4 Chemistry Flashcards Quizlet
 Histology Of Porcine Kidney Cortex Description Glomerulus Histology Of
Histology Of Porcine Kidney Cortex Description Glomerulus Histology Of
 Ap Biology Chapter 3 Reading Guide Flashcards Quizlet
Ap Biology Chapter 3 Reading Guide Flashcards Quizlet
