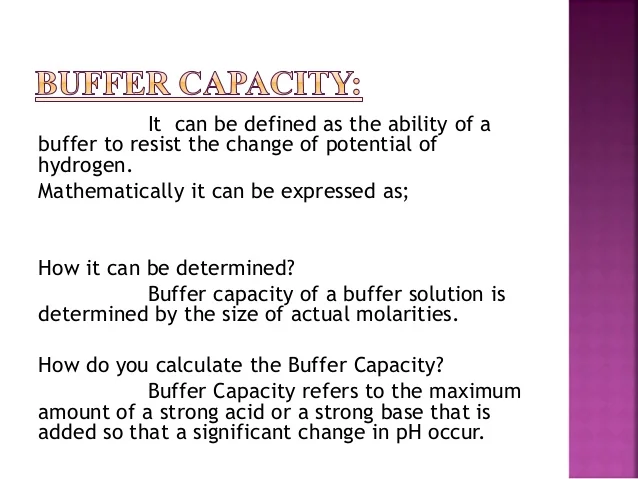
What Does Buffer Capacity Mean
Solutions with higher amounts of weak acid have higher levels of buffer capacity when adding a strong base. In streaming audio or video from the Internet buffering refers to downloading a certain amount of data before starting to play the music or movie.
What Is The Formula For Buffer Capacity Quora
In its simplest definition a buffer zone is an area between two or more adversarial powers whose function is to physically separate them.

What does buffer capacity mean. Having an advance supply of audio samples or. An example of a buffer solution is bicarbonate in blood which maintains the bodys internal pH. Buffer Capacity Buffers are characterized by the pH range over which they can maintain a more or less constant pH and by their buffer capacity the amount of strong acid or base that can be absorbed before the pH changes significantly.
It is a unitless number. The greater the buffer capacity the greater is the ability to absorb acid or base with little change in pH. Buffer capacity β is defined as the moles of an acid or base necessary to change the pH of a solution by 1 divided by the pH change and the volume of buffer in liters.
Buffering Definition In manufacturing the concept of buffering is defined as maintaining enough supplies to keep operations running smoothly. So to give a more clear definition buffer capacity may be defined as the quantity of a strong acid or strong base that must be added to one liter of a solution to change it by one pH unit. A buffer resists changes in pH due to the addition of an acid or base though consumption of the buffer.
Where n is some equivalents of added strong base per 1 L of the solution. Solutions with a weaker base have more buffer capacity when adding a strong acid. The buffer capacity equation is as follows.
Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes. It is thus conceived as an instrument. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
Note that the addition of n moles of acid will change the pH by the same value but in the opposite direction. Buffer solution measures a given soils reserve acidity or buffering capacity. Buffer capacity is a measure of the efficiency of a buffer in resisting changes in pH.
Buffer capacity is the amount of acid that buffers are capable of absorbing prior to breaking the capacity for adding strong acid. High buffer capacity means it can neutralize more moles of acid or base and keep the. Buffer capacity is a property of a buffer and it tells you how much acid or base you can add before the pH starts changing.
A buffer index can be calculated and then used to compute a lime rate needed to achieve a desired pH level. Buffer capacity can be also defined as quantity of strong acid or base that must be added to change the pH of one liter of solution by one pH unit. Buffer capacity A measure of the resistance of a buffer solution to pH change upon addition or removal of hydroxide ions.
Basically as your buffer capacity goes up which Im going to abbreviate BC as your buffer capacity goes up you can add more of your acid or base before the pH starts changing a lot. 2012 Farlex Inc. It is a unitless number.
Buffer index values for any given soil depend on the amount and type of clay and level of organic matter in that soil. A general buffer capacity estimate is 40 percent of the total sum of the molarites of the conjugate base and acid. The buffer capacity is a quantity in resisting the pH change at the time of addition of an acid or base.
It can be defined as follows. Conventionally the buffer capacity is expressed as the amount of strong acid or base in gram-equivalents that must be added to 1 liter of the solution to change its pH by one unit. Buffer capacity is a quantitative measure of the resistance to change of pH of a solution by camerino italy containing a buffering agent with respect to a change of acid or alkali concentration.
Buffering capacity is defined as the number of moles of strong base or acid needed to change the pH of a liter of buffer solution by one unit. Such definition - although have its practical applications - gives different values of buffer capacity for acid addition and for base addition unless buffer is equimolar and its pHpK a. Buffer capacity β is defined as the moles of an acid or base necessary to change the pH of a solution by 1 divided by the pH change and the volume of buffer in liters.
An area of land designated for environmental protection. The higher the acid concentration of the buffer then the buffer capacity will be higher as well. A buffer resists changes in pH due to the addition of an acid or base though consumption of the buffer.
These supplies often include the raw materials needed for production and also the inventories of finished products waiting for shipment. The buffer capacity can also be defined as the amount of mole of strong base needed to change the pH of 1 L of solution by 1 pH of unit.
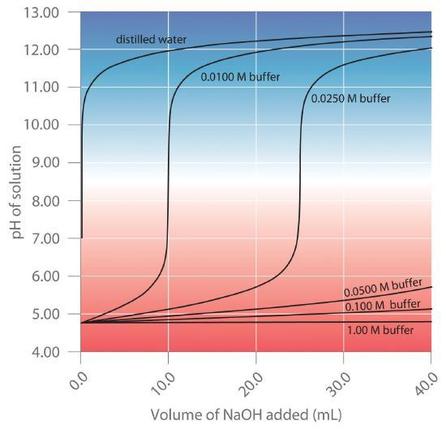 8 9 Buffer Capacity And Buffer Range Chemistry Libretexts
8 9 Buffer Capacity And Buffer Range Chemistry Libretexts
 Buffer Capacity Buffers Titrations And Solubility Equilibria Chemi Solubility Chemistry Ap Chemistry
Buffer Capacity Buffers Titrations And Solubility Equilibria Chemi Solubility Chemistry Ap Chemistry
 Buffer Solution Its Characteristics Types And Preparations
Buffer Solution Its Characteristics Types And Preparations
 Buffer Solution Acidic Buffer Basic Buffer Animation Buffer Solution Electron Configuration Solutions
Buffer Solution Acidic Buffer Basic Buffer Animation Buffer Solution Electron Configuration Solutions
 Determining Solubility From Ksp To Find Molar Solubility S Substitute S Or 2s Representing Number Of Molecules In Chemistry Education Solubility Chemistry
Determining Solubility From Ksp To Find Molar Solubility S Substitute S Or 2s Representing Number Of Molecules In Chemistry Education Solubility Chemistry
 Nikon Dslr Buffer Capacity Comparison Nikon Dslr Nikon Dslr
Nikon Dslr Buffer Capacity Comparison Nikon Dslr Nikon Dslr
 What Is Buffer Capacity Youtube
What Is Buffer Capacity Youtube
 Pin On Chemistry Acids And Bases
Pin On Chemistry Acids And Bases
 Buffers And Henderson Hasselbalch Chemistry Khan Academy Khan Academy Chemistry Henderson
Buffers And Henderson Hasselbalch Chemistry Khan Academy Khan Academy Chemistry Henderson
 Ocean Chemistry Acidification Time Scavengers
Ocean Chemistry Acidification Time Scavengers
 What Is Buffer Capacity And What Is The Condition For Maximum Buffer Capacity Youtube
What Is Buffer Capacity And What Is The Condition For Maximum Buffer Capacity Youtube
 What Is Kraljic Matrix Definition Model Example In 2020 Business Process Matrix Definitions
What Is Kraljic Matrix Definition Model Example In 2020 Business Process Matrix Definitions
 Period Science Flashcards Science Student Science Facts
Period Science Flashcards Science Student Science Facts
 Digital Kemistry Best Chemistry Animated Blogs What Is Valency In Chemistry Definition Example 11th Chemistry Chemistry Biochemistry
Digital Kemistry Best Chemistry Animated Blogs What Is Valency In Chemistry Definition Example 11th Chemistry Chemistry Biochemistry
 Transcription Science Flashcards Science Student Science Facts
Transcription Science Flashcards Science Student Science Facts